OdishaPlus Bureau
Human clinical trials of the country’s indigenously developed COVID-19 vaccine named ‘COVAXIN’ is set to begin this week at a Bhubaneswar-based institute, one of the 12 centres selected by the ICMR for conducting Phase I and II of the process.
The human trials of BBV152 COVID Vaccine or Covaxin will begin on Wednesday in a special laboratory set up at the Institute of Medical Sciences and SUM (IMS & SUM) Hospital, in accordance with the protocols laid down by the Drugs Controller General of India (DCGI).

Dean Gangadhar Sahoo on Monday inaugurated the special laboratory named Preventive and Therapeutic Clinical Trial Unit (PTCTU). “All necessary arrangements have been made for conducting the human trials. We expect to start from Wednesday,” said, Dr. E Venkat Rao, the principal investigator of the trial process and professor of community medicine at the hospital.
Describing the new PTCTU as the first such “dedicated human trial unit” in Odisha, Rao said it would facilitate clinical research in the future. “Some people have already volunteered to participate in the trial”, he said.
Several research organizations from across the country and outside have been eagerly waiting for such a unit to generate evidence in clinical research, based on factors such as the local population.
Equipped with all modern amenities, the PTCTU would focus on clinical trials involving preventive and therapeutic aspects such as vaccines, immunoglobulins, preventive practices, chemoprophylactic drugs, educational/behavioral interventions, and interventional agents or drugs.
People willing to volunteer for the vaccine trial can contact the institute. All details are displayed on its website. The center will go through the profiles and conduct a thorough screening to choose the suitable ones.”We will screen the prospective volunteers and thoroughly evaluate their health condition before administering them the vaccine,” Rao said.
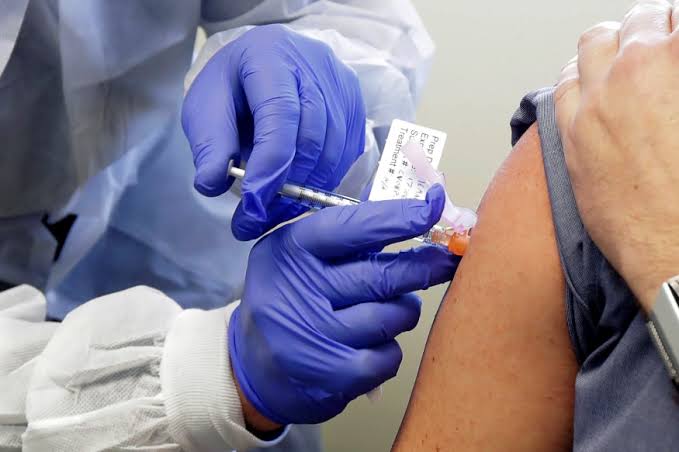
Healthy volunteers aged between 18 and 55 years having no co-morbid conditions or COVID-19 history would be eligible to participate in the clinical trials.
The trial center will conduct physical and laboratory tests, in accordance with the ICMR protocol, and counsel the volunteers who meet all criteria about the possible risks and obtain their consent before initiating the process, Rao said. All four phases of the clinical trials involving human subjects would be conducted at the center.
“We are committed to the maintenance of quality, ethics, patient safety, and confidentiality at the highest level. We conduct clinical trials involving investigational drugs or molecules approved by the DCGI and the Central Drug Standardisation Control Organisation (CDSCO),” Rao noted.





























